It’s time to treat Non-24 with HETLIOZ® (tasimelteon)
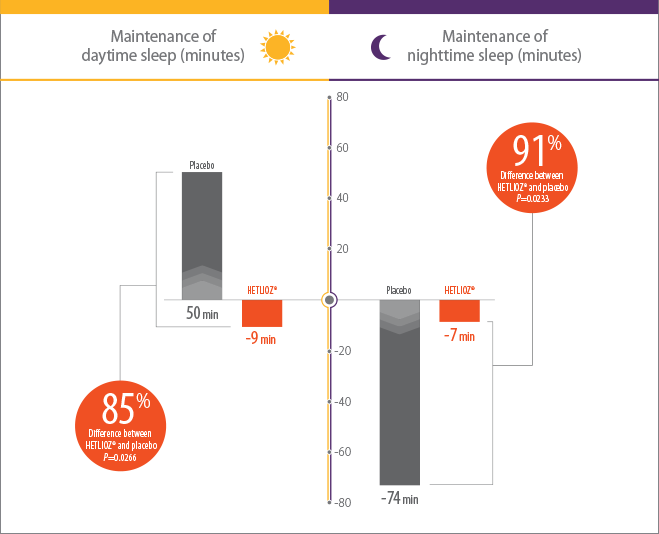
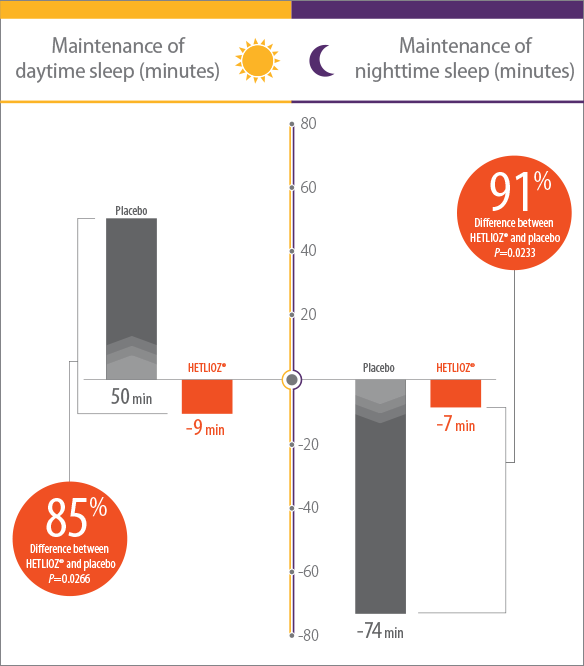
In RESET, a randomized withdrawal study of the safety and efficacy of tasimelteon, HETLIOZ® maintained significant decreases in daytime sleep duration and increases in nighttime sleep duration, as compared to placebo1
- Patients were treated with HETLIOZ® (tasimelteon) and then randomized to receive placebo or continue treatment with HETLIOZ®1
Study design—RESET: Patients who received HETLIOZ® and had entrained melatonin circadian rhythms (N=20) were randomized in RESET to receive placebo (n=10) or continue treatment with HETLIOZ® 20 mg (n=10) for 8 weeks. Entrainment is the calculated time of peak melatonin levels occurring at approximately the same time of day.1
RESET, Randomized Withdrawal Study of the Efficacy and Safety of Tasimelteon.
HETLIOZ® clinical trial program: 2 pivotal, Phase 3, multicenter, randomized, double-masked, placebo-controlled trials in patients who are totally blind with Non-24 1,2
- Efficacy assessments were based on the 25% of nights with the least nighttime sleep and the 25% of days with the most daytime sleep1
- Patients were more symptomatic when sleep-wake cycles were most misaligned with the 24-hour day1
Study 2: RESET2
- HETLIOZ® patients with entrained* melatonin circadian rhythms were randomized in RESET2

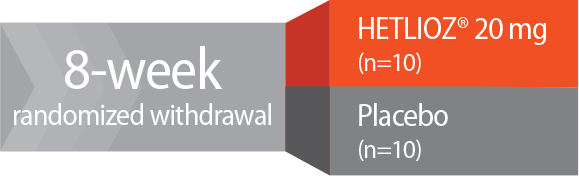
*Patients in whom the calculated time of peak melatonin levels occurred at approximately the same time of day.1
References: 1. HETLIOZ® [prescribing information]. Vanda Pharmaceuticals Inc. 2014. 2. Data on file. Vanda Pharmaceuticals Inc. 2014.
![HETLIOZ[R] (tasimelteon) capsules 20mg](/assets/header/header-logo-ed4a0eafea0a6d3ceec1cd9c4d717dea66814d23c9da61298b3f9181ff9546a6.jpg)


